Perhaps you have heard before about the CO2 drop checker. Or perhaps not, but it is quite probable that you did not get the full idea of what is telling you and how interpret it.
This article is thought to introduce this useful device to those who want to use CO2 in the aquarium and they do not know how to control its dosage of the tank. It will be also very useful to clarify how to read it, as well as discussing some of the most persistent questions about it.
Note that part of the discussion can become rather technical. I will try to keep it in reasonable levels, though, but I will include some sections that can be very tricky for those who have not been involved in Chemistry education. As a traditional seal of The Living Tank, I have decided to include that, so people can see this is not just "another interpretation" but just the valid interpretation that should be considered.
What is a CO2 drop checker?
Well, it is a fair question if you never heard before about it. In planted tanks or aquascapes with CO2 injection, the hobbyist needs a way to control the amount of CO2 that is inside the aquarium. Blindly doing so could have very negative effects, like:
- Suffocating your fishes if you are adding too much CO2.
- Getting a too low pH in the water, which can cause harm to the plants, too.
- Not reaching the desired levels and favoring the appearance of algae.
- Not getting the plants growing well due to a lack of CO2.
The best option would be to include a CO2 probe in the list of your equipment. However, they are expensive and also require supervision, maintenance, and calibration. That means that its usage is good for labs or for aquarium centers but not much for the hobbyist. Other alternative is to use kH and pH measurements to guess the maximum CO2 levels that can be present in your tank in the moment of the testing. However, test are not fully precise, the kind of tables (I show one later, in the Annex section) used for these analysis are not always well interpreted, and what is more important, one cannot be testing the full day or everyday just to guess if the CO2 levels are right.
 |
| Fig. 1: Example of drop checker. |
Because of all the above, best option is the drop checker device, focus of this article. The device itself is quite simple: A glass recipient able to keep a small chamber of liquid separated from the tank by a chamber of air. This split of spaces is critical for the drop checker to work well, so it is not casual, There are many variants of these drop checkers but the principia behind all of them is the same.
Most models include suction cups to hold it into the aquarium. Other models are thought to hang over the glass, with the bulb outside of the aquarium for an easier observation, and the other extreme bent over to fall inside the water of the tank.
The range of price of this simple object of aquarium can be huge, but generally, spending more money does not make them more accurate, and the cost usually involves different quality of glasses and/or more complex designs. In general, I would not advise to spend too much money on them, unless you can afford it and/or prefer more neat designs. The right message here is, nonetheless, that this can be obtained by just a few dollars, and can make a huge difference when managing the aquarium.
All the drop checkers work with an indicator solution. Such solution is just a pH indicator, more specifically known as Bromothymol Blue. The substance is sensitive to pH changes in the solution that induct into it a change of colour. It is the change of colour what helps to track the CO2 levels with the drop checker, so essentially, the device is able to monitor CO2 by changes in the pH of the solution in which the indicator is. This solution is always put into the bulb of the indicator, as in Fig. 1, and will work for long time, so it requires a very low maintenance in terms of money and time.
What are the principles behind it?
The Change of Colour
As already pointed out, the indicator is sensible to changes in the pH within the solution in the bulb. Bromothymol Blue is a molecule that varies its optical properties as result of the change of proton (H+) concentrations in the solution. I will explain in further detail this later, but essentially, the molecule changes from deep blue to bright yellow, depending on the state of a radical that forms part of its structure.
When we see the indicator as green is because there are so many yellow molecules as blue molecules (yellow + blue = green). This circumstance happens exactly at pH = 7.1. When the pH increases over such amount, the portion of blue molecules increases, becoming firstly in a dark green, later in a bluish green, light blue, blue, dark blue, with all molecules in blue form from pH = 7.8 or higher. On the other hand, when pH reduces, there are more yellow molecules than blue, which provides firstly a yellowish green colour to the solution that turns out fully yellow when the pH is 6.0 or below.
 |
| Fig. 2: Colour scale of Bromothymol Blue vs pH. |
The colour of a given molecule is related in how light interacts with it. In general terms, there are three ways in which light acts with matter:
- Reflection of the photons
- Absorption of the photons
- Transmittance of photons
The first one refers to photons of a given "colour" which just are unable to cross through a given substance and are not absorbed, either. This is the light reflected by objects, and what we see. The absorption are those photons that do not cross through the substance and are captured by atoms and molecules on it. These are the colours that we miss in the substance. In other words: the colour we observe is, from the white spectrum of light, those colours that are reflected in major proportion than the ones that are absorbed. The third property is a bit more complex to explain but relates to those photons that are nor absorbed neither reflected. The substance is just permeable to them. In some cases, this has also some effect in how the light is after leaving the object on the other side, but that is not relevant here.
In our case, what we observe is how light is reflected by the Bromothymol Blue molecule. This reflection varies because the disposition of atoms in this component change depending of pH. The process affects only to a small part of the molecule, but enough to produce a deep change of colour.
 |
| Fig. 3: Molecular change in Bromothymol Blue when crossing pH 7.1 |
Figure 3 shows this change. This is not fully obvious but let's simplify this by telling that, at pH above 7.1, this indicator tends to release some protons to the solution, which forces the shape. Below such pH this process tend to be in the opposite way, so getting protons from the solution. Depending on these protons, the shape changes and the optical properties of the molecule also vary, which causes the variation of colour.
The Change of pH
Now, we know why the colour changes, which is due to changes in the pH in the solution of the bulb. But then... what causes the changes of pH in the bulb? Well, this is the big question and the most important one. I will try to explain it in plain words by now.
The CO2 flux
As mentioned before, the drop checker does not connect directly the indicator solution with the water of the aquarium. This is for obvious reasons: otherwise the liquid will mix with the water of the aquarium and you would have no indicator to look at. To avoid that, a gap of air is created by design in the drop checker, which avoids this problem. However, there is a second and critical reason for it: the drop checker also avoids the contact of the solution with the atmosphere of the room. This is very important, as prevents the solution to be affected by the CO2 levels in the room, or being more precises, by the relative low levels of CO2 of the room, which would provide us with a wrong reading.
If not in contact with the water, how does the pH change then? It happens thanks to the air gap between the aquarium and the solution, which transfer CO2 between the two mediums. That part of the drop checker is in equilibrium with both solution and water of the tank. By equilibrium I mean that the air there will get different CO2 concentrations, depending on the amount of CO2 dissolved in both indicator solution and aquarium. As the volume of the water in the tank is thousands or even millions of times larger than the volume of the indicator solution, it is possible to assume the CO2 of the air gap will be related to the concentration of the gas in the aquarium.
At this point, it is worth to mention that the CO2 we are injecting into the tank does not stay there, but tends to leave the water because we add more CO2 than present in the air of the room. These difference of concentration, favors the flux of CO2 from the water to the air, by the Henry's law principle. Such law tell us that any air-water interface will have a flux of a given gas that will go towards the water when the concentration of the gas in the air is larger than the one in the water, and this one has not achieved saturation in the water. It also tells us that the gas will leave the water and go to the air when the concentration in water is saturated and/or higher than the concentration in the air.
This idea also works for the air gap in the drop checker. In this case, the equilibrium is a bit more complex, as there are two interfaces: [aquarium water]-[air gap]-[indicator solution]. Regardless, the mechanism is easy to understand: When we increase the CO2 concentration in the aquarium, a part of it will pass to the air gap, process explained by the mentioned Henry's law. The air gap will increase then its CO2 concentration, until such a point that the levels are higher than the amounts in the indicator solution. At this point, part of the CO2 of the air gap goes to the indicator solution. On the other hand, when the levels of CO2 diminish in the tank, the air gap loses CO2 towards the aquarium, and when the CO2 levels in the air gap are low enough, a flow of CO2 starts from the indicator solution to the air gap.
The speed in which this process takes place depends on the relative volumes of aquarium and indicator solution, but as they are massively different, the equilibrium is achieved again after a few minutes following any change in the conditions.
There are some proportions linking the process, but I will not go further into that now. This kind of interaction is rather complex and we could talk for hours in order to fully understand how works. There are, however, two main points to consider with respect the carbonates:
Because of the above, carbonates in he indicator solution are strongly affecting the readings in the drop checker. Nevertheless, if we assume that the pH changes in the drop checker are only due to the CO2 levels, which is a fair assumption, then the CO2 concentration can be determined as
where dkH are the degrees of carbonate hardness. We can play a bit with this equation, mathematical speaking, by transforming the equation to see what is the change of pH associated to a given CO 2 level:
And then,
Despite of the numbers, the important point here is that, as you can see, the hardness and the CO2 concentration have opposed signs, with kH increasing the pH and CO2 reducing it. This is the key point of the whole thing, and it has our attention in the rest of this article.
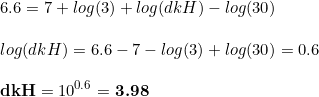
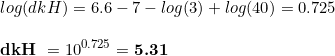

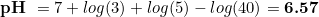
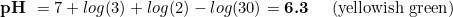

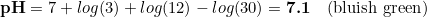
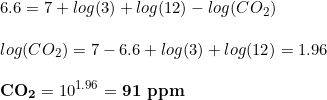
It is also very relevant to tell that the equation I have used to explain the relationship between pH, kH and CO2 levels only is valid if we assume that all the alkalinity in the indicator solution is due to carbonates.
Alkalinity can be a tricky concept but the easiest way to understand it is like the capability of a solution to absorb or neutralize protons. This can be due to carbonates, but there are more substances able to impact into it, like phosphates, or even ammonia (NH3 becomes into NH4+ at low pHs). These other molecules are present in aquariums and, in general terms, their contribution to alkalinity is much less than carbonates, but they will deviate the results. This is other of the reasons why is not good idea to use water of the tank for the drop checker.
Other factor to consider is the temperature. Most of the equilibrium that have been shown here are under normal conditions (1 atm of pressure and 25º C). Pressure can be considered usually like that but temperature can differ depending on many things. Most hobbyist try to preserve their tanks with about 25º C, but this could be not possible at every country, and sometimes other external factors (like radiators in the houses) can have impact. It is a good idea to minimize this effect by putting the drop checker just where the thermometer is located, which at the same time, it should be in the opposite side of the tank where the heater (if any) is located, or from the water outlet if using inline heater or filters with built-in heaters. In general terms, testing conditions should be preferred at such temperature. No significant changes will happen by small variations of it, but large departs can appear if we allow the temperature to increase or go down more than 3º to 5º.
The intensity of colours as shown in Fig. 2 can also vary depending on the concentration of the indicator in the drop checker, which also will depend on the brand. If too diluted, changes in colour can be difficult to see, and the same goes if too concentrated. In general lines, is much better to follow the instructions of the manufacturer when dosing indicator solution.
This is also something to take into account when deciding the kH of the drop checkers, as some manufacturers already include the indicator in a solution with a given kH (usually 4 dkH). In order to avoid errors, best idea is to check it in the user's instructions and if not available, in the website of the brand. In other cases, the manufacturer expressively ask the user to fill it with a dkH solution that you can buy separately or make it yourself. I explain later on how to prepare a solution for a given kH.
If you do not find the details of the dkH in the indicator solution coming by default, then you have the following options:
So, at this point, I have explained what the drop checker is and how it works. I have also pointed out how we can use it in our benefit to measure desired levels of CO2, and also indicated some of the issues and errors we can have whit it.
Now, I have prepared a list of do and don't to help in its use, as well as some tables of reference for easier use than already existing ones, which commonly offer incomplete information.
You can get the full size one by clicking on the image. Alternatively, you can download it in PDF format here. Please, make sure you refer to The Living Tank whenever/wherever you use or promote this chart.
In all these cases, having the knowledge to prepare it by yourself is the solution.
In the equation, V is the volume in litres of your solution, and R must be selected depending on the chemical you are using to prepare it.
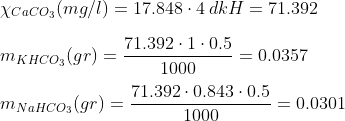

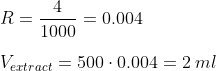
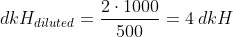
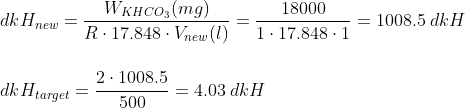

The speed in which this process takes place depends on the relative volumes of aquarium and indicator solution, but as they are massively different, the equilibrium is achieved again after a few minutes following any change in the conditions.
The effects on pH
So now we know how the CO2 are balanced between aquarium and drop checker. The link to pH is related to the effects of this gas dissolved in water, which happens to be H2CO3, an acid. As any acid, its solution in water release protons (H+), hence, reducing the pH. Note that pH is understood ad the ratio of concentrations of ions hydroxyl (OH-) and protons. The largest the amount of OH- ions, the higher the pH, and the larger the amount of H+ ions, the lower is.
Thus, this "injection" of CO2 into the indicator solution causes a reduction of pH, which is proportional to the amount of CO2. This is not fully a relation one-to-one because the formation of bicarbonate iones (HCO3-) when releasing a proton to the water it depends in both CO2 concentration and also the already existing concentration of carbonates (CO3(2-)).
| Eq. 1: Carbonates equilibrium |
In Equation 1 I show this equilibria in a simplified way. The main driver of it is the concentration of HCO3(-), which links carbonates with carbon dioxide. When pH is very low, CO3(2-) molecules tend to absorb protons to become HCO3(-). This phenomena has two consequences:
- Increasing of pH by reducing the concentration of protons (H(+)).
- Preventing the dissolution of CO2 in water, as the carbonic acid molecule (H2CO3) is not much stable in water and tends to dissociate whether to HCO3(-) or to CO2, being the latter case the main one under this situation.
There are some proportions linking the process, but I will not go further into that now. This kind of interaction is rather complex and we could talk for hours in order to fully understand how works. There are, however, two main points to consider with respect the carbonates:
- CO3(2-) molecules are a basic pH buffer, which means that they compensate part of pH reductions in the solution by absorbing protons and becoming into HCO3-.
- By doing so, they also limit the amount of CO2 that can dissolve in the solution, what means that equilibrium in the indicator solution is achieved at higher pH than it would if no carbonates were there.
Because of the above, carbonates in he indicator solution are strongly affecting the readings in the drop checker. Nevertheless, if we assume that the pH changes in the drop checker are only due to the CO2 levels, which is a fair assumption, then the CO2 concentration can be determined as
| Eq. 2: Relationship between carbonate hardness, pH and CO2 concentrations. |
| Eq. 3: Logarithmic form of Eq. 2. |
And then,
| Eq. 4: Obtention of pH ad result of carbonate hardness and carbon dioxide concentrations |
Despite of the numbers, the important point here is that, as you can see, the hardness and the CO2 concentration have opposed signs, with kH increasing the pH and CO2 reducing it. This is the key point of the whole thing, and it has our attention in the rest of this article.
The Effect of the dKH solution
Many of you are already aware of this, but there is a heated discussion about whether the drop checker must have a fixed carbonate hardness (kH) in order to show the right results or not. The question is fully valid, as per the above description, kH is critical to determine the change of pH and the colour the indicator solution will show.
With the modification of the equation that we did, the answer is more clear. Fig. 2 shows that green colour in the drop checker is reached when pH is 6.4-7.0, roughly speaking. If we want to use green colour as reference of "OK" levels of CO2, then we need to apply the mentioned equation to know what carbonate hardness we shall have in the indicator solution in order to achieve the wished CO2 concentrations.
Most hobbyist target CO2 levels of around 30 ppm (mg/l), so we are going to consider such amount. At the same time, we are going to assume that our desired colour corresponds to a pH of 6.6. Doing so, we can start to make some numbers:
So there you go: dkH = 4 is the one you want in the drop checker to ensure the green colour matches with the CO2 levels, as most sites recommend to have.
Nonetheless, the good lesson here is that you have now some control over this, and by adjusting the kH values in the indicator solution you can target other CO2 levels.
For instance, some hobbyist not interested in keeping fishes or having them in few numbers would like to boost CO2 to help the plants. Common CO2 values in this sense are 40 ppm. Considering that, what dkH shall you have in the drop checker? The target pH remains unchanged, so the equation is now:
And what if I have not many plants and wanted to track CO2 concentrations up to 20 ppm? No problem:
These are the exact dkHs that will make full green the indicator solution for a given CO2 concentration.
Note that, however, for many people is difficult to get exact values of kH at home, and that could be interpreted as a source of significant error due to the exponential nature of the formulae.
Let's exemplify this by considering the case of the targeted 40 ppm of CO2. In the computation, the exact kH we need is 5.31. This is difficult to get, so most people would round this to 5 dkH. What is the impact of this small reduction of kH?
So the lack of precision is not dramatic, especially considering that green colour is produced at a range of pHs, and 6.57 definitely falls inside.
However, more problematic is those people who are using the water of the aquarium to fill the drop checker, as that can lead to really wrong lectures, especially considering that discriminating tonalities of green can be difficult. Some people are fan of using low kH values at the tank in order to favor the existence of more CO2 (aq) rather than HCO3-. As CO2 is injected, pH falls, which makes more difficult the formation of HCO3-.
Supposing that kH=2 in the tank (very low) and that the drop checker is filled with the same water, then, the pH of the drop checker (and hence the colour) when CO2 levels are of 30 ppm is:
Most hobbyst could tend to reduce then the CO2 flux to reduce it a bit, By doing so, we got concentrations below 30 ppm:
So half the concentration!
On the other hand, many hobbyist have high carbonate hardness levels in tap water. This can be quite problematic if you use it in the drop checker. Considering a common kH=12 in tap water:
Most hobbyist could tend to increase the CO2 flux in order to get a green colour, but then they will putting CO2 levels over 30 ppm, and if not care with this, fishes can suffocate. In this last example, the CO2 concentration turning green the drop checker is
So three times the targeted CO2 levels!
From these results is derived that best option is to use a known carbonate hardness in the drop checker. Otherwise, we risk to have wrong measurements, and in the case of especially hard waters, we can risk killing the fishes by adding too much CO2. The results also tell us that small variations in the colour can imply significant changes in CO2 levels. That means that the drop checker can be a bit difficult to interpret sometimes, and obviously not very good option for those suffering colour blindness or chromatic aberration.
Further considerations
There are some other factors or questions to be answered that I cover now. They are less important than the right understanding of how the drop checker works, but nonetheless they should be considered to ensure a right reading.
Alkalinity
It is also very relevant to tell that the equation I have used to explain the relationship between pH, kH and CO2 levels only is valid if we assume that all the alkalinity in the indicator solution is due to carbonates.
Alkalinity can be a tricky concept but the easiest way to understand it is like the capability of a solution to absorb or neutralize protons. This can be due to carbonates, but there are more substances able to impact into it, like phosphates, or even ammonia (NH3 becomes into NH4+ at low pHs). These other molecules are present in aquariums and, in general terms, their contribution to alkalinity is much less than carbonates, but they will deviate the results. This is other of the reasons why is not good idea to use water of the tank for the drop checker.
Temperature
Colour intensity
Default dkH in the indicator solution
If you do not find the details of the dkH in the indicator solution coming by default, then you have the following options:
- Drop an e-mail to the brand and see if you get an answer (unlikely).
- Do not use the indicator coming with the drop checker by default, and buy another indicator from a brand that offers the information (probably the easiest way).
- Make a test to check this.
Maintenance
A frequent question is how often one has to change the indicator solution in the drop checker. Under my experience, indicator solution can last forever, as it just suffer some slow degradation coming from light. That degradation can take months to be noticed, and usually makes the liquid to get a different colour (brown/black). If you observe this in the bulb, then is time to change it.
Nevertheless, there are more reasons to change it more often, mainly related to the fact that the solution in the bulb will not keep a stable dkH value all the time. The reason is evaporation or gain of water in the drop checker. As it has so small volume of liquid, tiny variations in the amount of water can affect to the carbonate hardness, and then, altering the results. A good rule of the thumb is to renovate the indicator solution every three months.
The Drop Checker User Manual
So, at this point, I have explained what the drop checker is and how it works. I have also pointed out how we can use it in our benefit to measure desired levels of CO2, and also indicated some of the issues and errors we can have whit it.
Now, I have prepared a list of do and don't to help in its use, as well as some tables of reference for easier use than already existing ones, which commonly offer incomplete information.
What do I need?
The following list of items is recommended in order to properly use the drop checker:
- A glass drop checker.
- Indicator solution for it (usually comes already with it).
- Distilled or RO water.
- A scrubber to clean chalk and algae.
- A soft acidic solution to dissolve persistent dirt.
How do I prepare it?
1. Clean well the drop checker. For that purpose, use the distilled or RO water and wash it but not adding any kind of soap or other products, as that could alter the results.
2. Check in the instructions or website of the manufacturer what is the dkH solution of the indicator solution. Some manufacturers alternatively provide a pre-made 4 dkH solution, which obviously means the indicator has no dkH.
3. If the indicator solution is pre-mixed and has already some dkH, then fill the drop checker as indicated by manufacturer, but be aware that your readings will be determined by the kH already in the solution (which usually is 4 dkH). In this case, we have not much maneuver to try to track different CO2 concentrations, apart from trying to match the colour associated with it. I provide charts later on that can help into that. Nonetheless, targeting 30 ppm of CO2 is enough for most people.
4. If is not pre-mixed, then fill the drop checker with prepared water as explained in the instructions at the dkH level you want, and following instructions, add the indicator.
Important note: Never use water form the aquarium or from the tap for this purpose; always fill the drop checker with a pre-made dkH solution, or with your own but using RO/distilled water. In those cases in which indicator is pre-mixed, just follow the manufacturer's instructions.
How to install it
Depending on the design, your drop checker will hung from the glass panel or it will be attached to it using a suction cup. Follow the specific instructions of the manufacturer if you do not know how to place it. However, follow the next tips for the installation:
1. Put the drop checker far away from any heating source in the aquarium, so avoiding the heater, or when corresponding, the outlet if the heating is done inline or in the filter. It also helps not to put it just below the lightning unit nor close to any radiator in the proximity of the aquarium (if any).
2. Water flow matters. For the drop checker to offer measurements of the CO2 in the tank that are meaningful, a good mix of water must be present. Good water circulation is critical for the planted aquarium, anyway, as many problems with CO2 come from lack of mixing. You can use the drop checker at different locations of the tank to see if the water is well mixed in terms of CO2, but in general terms, it is good to put the drop checker in an area with a good circulation of water so you have a better result of the amounts of CO2 being injected in the tank.
Important note: Never put the drop checker in a dead zone, in terms of water circulation, as then it will not provide readings accordingly with the CO2 you are injecting.
How to read it
1. Allow some time to the drop checker get the right equilibrium. This will be faster or slower depending on the design of the drop checker and other conditions like temperature and volume of the bulb. A safe interval of time is 3 hours before trusting the measurements.
2. Check always the colour of the drop checker against a white background for a good reading, or use a mix of reference. There are some drop checkers that offer this option by having two or more bulbs. Meanwhile they are better for this purpose, they are also much more expensive.
Important note: If you extract the drop checker from the tank to see better the colour, be aware that the air gap will be filled with air from the room and then the drop checker will start to adjust to the concentration of CO2 in the room. Because of that, it is strongly recommended to do a fast reading and replace the drop checker in the aquarium as soon as possible. It is quite probable the indicator will become blue even after that, and it will take sometime for the reading to be good again (a few hours as said before).
3. Contrast the color against a chart of reference to know the CO2 concentrations you have. I have prepared a chart that you can use for this purpose, easier to use that most ones already available on internet (in this article is the one I recommend, see Annex below).
Important note: As explained in this article, results will vary depending on the dkH. Make sure you know the dkH of your drop checker! Otherwise, interpretation of the colours will be misleading.
How to maintain it
1. It is good practise to change the indicator solution in the drop checker at least onece every three months, or at any moment in which the liquid becomes brown or black.
2. Glassware tend to accumulate carbonates and green algae. For a neat reading and for cosmetic reasons, it is convenient to remove the dirt. A non-metallic scrubber is enough to clean them. Persistent chalk can be removed with a soft acid solution (e.g. lemon juice or wine vinegar). Do not forget to wash up well the glass with water to remove any acid or remaining particles.
Important note: When cleaning the glassware, try to use always RO/distilled water for a final wash up, in order to eliminate any substance that could alter alkalinity or pH of the indicator solution.
Conclusion
As you can see now, the drop checker is a very useful device. However, a proper understanding of the principia behind it is necessary in order to avoid errors and understand what is telling us. A good set up of the device is critical to ensure we get the right measurements.
Nevertheless, as any colourimetric technique, reading the colour by eye will be always imprecise, so it is unlikely we will get accurate estimations of CO2. Because of that, the drop checker will always just be a guidance to the CO2 levels in the tank, and targeting a exact concentration will be difficult.
However, plants in nature live with varying CO2 concentration in the water, which means that they also accept a range of values. By minimizing the common errors done during the setup of the drop checker, we can obtain a reasonable guess of the CO2 concentrations in the tank, situation much better than not having any idea or, even worse, having really wrong readings!
Additionally to the information above, I have also produced a pH/kH chart that includes the colour of the drop checker, less confusing than other available on internet. I have also added an explanation about how to produce your own dkH solution. All these details are in the Annex that you can find below.
And that's it! I hope you enjoyed the article and found it useful! Please, feel free to comment and share this article that I think can be very useful for the community.
And of course, if you had any question or query, please, do not hesitate in contact me through the Contact section of the website.
All the best,
The Living Tank
Annex
This section includes also miscellaneous information useful to work with the drop checker.
The CO2 chart and its interpretation
As deducted from the article, the right levels of CO2 are depending a set of variables not limited to the colour of the drop checker. In order to help in this, I have created a new chart easier to use and interpret than already existing one, which usually are a bit misleading. The chart is as follows:
 |
| Fig. 4: Chart relating drop checker colour with CO2 levels, depending on kH of the indicator solution |
You can get the full size one by clicking on the image. Alternatively, you can download it in PDF format here. Please, make sure you refer to The Living Tank whenever/wherever you use or promote this chart.
Why use this chart?
The main difference respect existing charts is that this one links the colour of the drop checker with the CO2 concentrations for a given kH. General charts are provided to know the CO2 concentrations by measuring kH and pH of water in the tank. However, as explained in this article, doing so can introduce large errors in the estimations. As a result, some hobbyist could be facing problems to regulate CO2 levels as they could be mistaken the values.
How to read this chart
The principle of this chart is easy. You only need two things to find out your current CO2 levels:
- The kH or carbonate hardness (in dKH) that the solution of your drop checker has.
- The colour of your drop checker.
Known the kH, you get the CO2 concentrations in ppm by crossing that column with the row associated to the colour of the drop checker. As you can see, the same colour has an associated different CO2 concentration depending on the kH value and the range of variation is quite large in all the range of kH values. This is why knowing kH in the indicator solution is so important.
The table is useful in more aspects. For instance, it is possible to play with the kH to force a given colour being associated to a CO2 concentration that we want to have or we want to put as limit. For example, if you want to have a colour as the one obtained at pH 7 and a CO2 concentration of 30 ppm, then the kH in the indicator solution must be 10 dkH.
Other option is to fix a kH and then play with the colour to get the desired CO2 levels. For instance, if you want to target 40 ppm instead of 30 ppm, and you have a fixed kH of 4 dkH in the
It can also be used to know CO2 levels by directly testing pH and kH of the water of the tank. However, as widely explained in this article, this can lead to errors, as pH in the tank is associated to many other chemicals and not only the CO2 and alkalinity, and the alkalinity can also be affected by other substances not related to carbonates, so I strongly advise against using the table in such a way unless you know what you are doing.
Preparing your own dkH solution
A common or frequent question is how to prepare your own dkH solution. Firstly, it is worth to say that there are a few brands selling the already-made solution, usually fixed at 4 dkH, so if you do not feel comfortable in Chemistry or you do not want to risk errors, then buying the product should be your option.
However, preparing your own solution can be quite convenient for a few reasons:
- It is cheaper than buying it.
- Finding the pre-made solution in the market is not always easy or possible.
- Perhaps you do not want to get a fixed 4 dkH solution, if your target of CO2 is not 30 ppm.
Materials
To prepare the solution, you will need the following materials and equipment:
- Distilled/RO water (better the former, as RO water sometimes has still chlorine).
- Potassium carbonate (KHCO3) or sodium carbonate (NaHCO3, baking soda)
- Precision scale.
- Glass recipient to prepare the solution and store it.
The basis of it
The first consideration you will need to do is: what dkH I want to have in my solution? Depending on that, numbers below can vary, reason why I explain here how to calculate how much KHCO3 or NaHCO3 you will need, so you can determine the amount yourself for any dkH you wish to obtain.
As you probably already know, carbonate hardness is measured in dkH. This unit what really represents is the concentration of carbonates and bicarbonates present in a given solution, if we consider that all them are in the form of calcium carbonate (CaCO3). More, specifically, 1 dkH is equal to 17.848 mg/l (ppm) of CaCO3.
Unfortunately, CaCO3 is practically non soluble in water, so it is not good idea to use it to prepare a solution with a given dkH. Somehow, we need to translate this to a soluble form of carbonates, in this case, KHCO3, because, as I will show later, simplifies the poblem, but works equally well for NaHCO3. To do so, we have to employ the molecular weights associated to both chemicals in order to pass from one to the other.
Step 1: Determine the concentration of CaCO3 you need for your target dkH. This is quite easy, as it is equal to the CaCO3 of 1 dkH multiplied by the desired dkH:
| Eq. 5: Determining CaCO3 associated to targeted dkH |
Step 2: Translate the concentration of CaCO3 into mass of KHCO3 or NaHCO3. This is done using the molecular weights associated to each chemical. Moreover, this is as easy as multiplying the previous value by the ratio of molecular weights between KHCO3 and CaCO3:
| Eq. 6: Computing the ratio between CaCO3 and KHCO3. |
So, as you can see, the advantage of using KHCO3 is that the ration R is equal to 1, which means that using CaCO3 or KHCO3 makes no difference. It is possible to use other substances, like NaHCO3 (sodium bicarbonate, or baking soda), but then the ration will not be equal to 1:
The way to do the translation is easy: Just multiply the resulting amount of CaCO3 for your targeted dkH by the ratio R above computed, and that for the volume of solution you wish to prepare (in litres) and divided by 1000 to pass from milligrams to grams:
| Eq. 7: Computing the ratio between CaCO3 and NaHCO3. |
The way to do the translation is easy: Just multiply the resulting amount of CaCO3 for your targeted dkH by the ratio R above computed, and that for the volume of solution you wish to prepare (in litres) and divided by 1000 to pass from milligrams to grams:
| Eq. 8: Obtaining the mass in grams of the chemical for the dkH solution. |
In the equation, V is the volume in litres of your solution, and R must be selected depending on the chemical you are using to prepare it.
Step 3: Known the amount of potassium or sodium carbonate you need, then proceed to weight it into the precision scale and dissolve it into the distilled/RO water. Make sure that you use the volume of water you are using now into the Equation 8 above described. It is very important to make sure the chemical is well dissolved so take your time to move the solution until no crystals of potassium o sodium carbonate are present. For this step it is better using a transparent glass or plastic bottle.
Step 4: That's it! Yo are ready to fill your drop checker with the obtained solution and add the indicator solution.
Example: Let´s say you wish to prepare 500 ml solution of 4 dkH. Following the steps indicated above, we have that:
So the amount of KHCO3 we need for 500ml of 4 dkH solution is of 0.0357, and if we want to use NaHCO3 instead, the amount is 0.0301.
One minute... these amounts in weight are very small!
Well, yes, you are right. In some cases, even with a precision scale measuring such weights can be tricky. Fortunately, there is a solution for that, which consist in a multiple dissolution process. The idea is to initially prepare a much more concentrated solution that allow us to measure the weight of chemical we need, and then dilute the solution. The only "counterpart" is that it requires more distilled/RO water.
Technically speaking, you can prepare a solution of a known concentration, and then compute how much of such solution you need to dilute in other volume to achieve the desired dkH. For the explanation purposes, I am going to assume you want to prepare 500 ml of a dkH 4 solution, but you cannot weigh anything below a gram.
Step 1: Determine the weight of KHCO3/NaCO3 you need for your desired dkH and volume of target solution, as explained above.
In our case, 500 ml of a 4 dkH solution. Following the example I put above, these amounts are 0.0357 and 0.0301 grams, respectively.
Step 2: Multiply the obtained weight by a factor large enough to make us possible the measurement in weight (rounded to the gram or to the precision of the scale you use). Remember this factor, as you need it later.
For example, 500. Doing so, and rounding to the gram, we would need 18 grams of KHCO3 or 15 grams of NaHCO3 in our example.
Step 3: Dissolve the chemical into 1 litre of distilled/RO water and determine the dkH in this solution. This is done as follows:
| Eq. 9: Computing new dkH for the concentrated solution. |
As we have multiplied by 500 the weight of the product, but doubled the volume of the solution, we have now 1 litre of 1000 dkH (4 dkH multiplied by 500 and divided by 2). Make sure the product is well dissolved in the water.
Step 4: Determine the volume of the concentrated solution you will need for the new diluted solution. This is done in two steps: a) Find the ratio of dkH between the target dkH and the one in the concentrated solution; b) Multiply the result by the volume of the target solution:
| Eq. 10: Determining the required volume of concentrated solution. |
In our example, the result would be:
So we will need 2 ml of the 1000 dkH solution.
Step 5: Using a syringe (you can get them in almost every pharmacy) , extract the indicated volume of the concentrated solution, and add it to a volume of distilled/RO water equal to the volume of solution you initially planned to make. Check the result is correct by doing:
| Eq. 11: Verifying the numbers. |
In our case, we take 2 ml of the 1000 dkH solution we obtained in first place, and then dilute that into 500 ml of distilled/RO water to prepare our 500 ml 4dkH solution:
We succeeded!
The advantage of this method is that reduces a lot the potential error of weighting the chemical. For instance, we used in the example 18 grams of KHCO3. What will be the actual dkH in the final solution?
This can be found by calculating the dkH that 18 grams would originate into the initial solution, and then use Equation 11 to know the final dkH. In the process, we need again the ratios of molecular weights between the chemical used and CaCO3. In our example we used KHCO3, so let´s keep it. The result will be then:
So, really close to our targe. In fact, even if we mistake 1 gram over the exact amount of chemical, the error would be still aceptable:
This method is very good to fix any problem with imprecisions in the measurements of amounts and/or volumes.
Hi, after reading your article I have some questions.
ReplyDelete1) My current tank has a PH of 6.6 when CO2 is off. My drop checker is green
when CO2 is off and yellowish green when it is on. So according to your article, I should
target a 1.0 drop in ph (Which is 5.6) to achieve 30ppm. I'm using a default 4dkh solution provided
by the vendor. My question is, how do I optimize my drop checker in a way that when my co2 is off it stays blue
and when it's on it turn green, instead of the green to yellow I'm experiencing right now.
2) Why am I experiencing this? Am I using the incorrect solution for my tank?
Tank Parameter
PH: 6.6 (When CO2 is off) Under 6 (When it is on)
KH: 2
Hi Daren,
DeleteMany thanks for reading this blog and asking questions. We are here precisely for that.
I think I understand the issue you face. Essentially, your drop checker does not get down of the comfortable green area when there is no CO2 injection and instead, gets yellowish when you turn on CO2.
Based in what you report, the issue is not the drop checker solution, really. Note that the drop checker is a separated environment and this is done at purpose, so that the change in colour obeys to a calibration targeting around 30 ppm based in a solution at 4 dkH. In other words, if it stays green when you are not injecting CO2, this is happening essentially because the equilibrium CO2 of the aquarium corresponds to concentrations of around 30 ppm of CO2 already. When you turn on the CO2 system, then it increases above 30 ppm, giving you the yellowish colour.
Please, note that the 30 ppm concentration that the drop checker measures goes in absolute value, whilst the associated pH change does not. This is important because essentially means that the solution in the drop checker, set at 4 dkH turns in the nice light green when the equilibrium of CO2 between drop checker and aquarium reaches around 30 ppm. But if your aquarium has a base level of CO2, the drop checker will still turn colour at the same point, so if you target a 1 pH unit drop, then you might be adding too much. This is the reason I think using that criteria of pH purely is risky, as just using a pH probe will not have into account the basal levels of CO2 in your aquarium, which in my view, it is a serious risk. As a general rule, drop in pH of 1 unit between CO2 injection on/off imposes a variation of CO2 of 10 times the basal level. If your aquarium is in equilibrium with the atmosphere, then 1 pH drop sets the CO2 concentration in gaseous form in the water from about 3ppm to 30ppm. BUT if your aquarium has a starting concentration higher than that, then a pH drop of 1 unit might imply a far larger swing of CO2 concentration. For instance, according to your parameters your basal level is of 15.1 ppm of CO2 (just check the colour chart for your kH and pH values with injection in off). So if you target a drop in pH of 1 unit when you inject CO2, then your CO2 concentration in the aquarium may reach 151ppm! So obviously is not the way forward. The colour of the drop checker is much better indicator because tells you if you are directly in the right range of CO2 concentration or not. This enables a finer control of the CO2 injection process than just measuring pH.
Now, the question really is about why your basal concentration does not get lower than 15.1 ppm when there is no CO2 injection. In principle, the most likely one is that there is not time enough, since you switch off the CO2, for the aquarium reach equilibrium with the atmosphere before the injection is switching on again. This happens quite often really if you have low level of surface agitation/aeration. I did not detail this much in the article, as it would have made it too long (even more!), but the transfers of CO2 between aquarium and air in the room may happen with a large range of time scales, being the main factor the agitation/aeration rate of the aquarium. The less agitation/aeration, the more time it takes for the CO2 to drop. In addition, quite often we switch off the injection no long before the lights are switched off too. This means that we also stop the main removal mechanisms of CO2: the photosynthesis of the plants on it. Moreover, many people forget that plants actually breathe, which means they generate CO2 too. And if the mass of plants is considerable, that amount is not precisely minor. No lights means no CO2 intake and instead CO2 production by the plants (and bacteria and other organisms), which if you combine with no enough agitation/aeration, means that CO2 concentration drops at significant slower rates than one might expect.
This is the reason why in planted aquariums are people who prefer to ensure right aeration/agitation at night period. This might be achieved by moving the lily pipes closer to the surface during the nights, so that there is an increased mixing of air and water in the surface, or by adding active aeration systems (e.g. air diffusers). For some time, Takashi Amano was favouring the latter, including timers that allowed to switch on air diffusers when the CO2 was switched off and vice-versa. I have found that this is perhaps too much aeration and causing pH swings that might be significant between day and night, especially at low kH values. For me the best option is just use the position of the lily pipes. The only reason why I really care about pH swings are the fish I have, but for those with no fish, this might be of no concern, or in some cases, there are fish species that are just able to manage well these swings. But usually I favour stability of the water parameters as much as possible. However, aeration/agitation might be a good idea for heavily planted aquariums or with a good amount of fish (or large fish) which will benefit of lower CO2 levels at night, to prevent pH in the light hours to get too low.
DeleteNote also that the indicator solutions do not last forever, and sometimes we can do silly mistakes, like washing the internal part of the drop checker with tap water instead of distilled/RO water, leaving mineral depositions of carbonates on it (i.e. altering the 4 dkH solution we put on it afterwards). An easy way to check if your drop checker works well is to get it out of the aquarium, and leave it outside for about 1 hr (to be sure) and then check the colour of the indicator solution. If there are no troubles, the solution should get blue as corresponding to 4 dkH in the table and the row where CO2 concentration is around 3 ppm for that dkH. If does not do this, then best course of action is to change the indicator solution in the drop checker and do the same to verify it solves the problem. If not, whether the solution is corrupted/outdated or you have to do a good cleaning of the drop checker (rarely the case).
Other aspect that might help is that the position of the drop checker in the aquarium does matter. Water in aquariums is not perfectly mixed; in fact there are a few reasons why is well the opposite. It is also a common problem that drop checker is set too close to where the CO2 is being injected, or directly in the trajectory of the flow of the outlet pipe, which may have also impact in the readings. A trick to ensure the reading is good is to change it of location in the aquarium several times over consecutive days, and monitor the colour. This does not happen if the is a good level of turnover in the filtration (good rate of flow of the filter respect to the volume of the tank). But if the filter does not mix well water, then you might experience these type of issues.
As final comment, as said before, there is really nothing negative in having a basal concentration close to 30 ppm. In my view, I always try to keep this stable. If I reach that point, I just adjust the CO2 injection to help keeping at 30 ppm during the day and to compensate for the loss due to the plants uptake. A slightly yellowish colour is not serious really, but you should get concerned if your drop checker really gest yellow. This require some test on pH for some days and some vigilance to ensure you adjust this as your biomass in plants grow, but besides of that, it helps to keep a stable pH which is largely preferred for fish.
Hope this helps to answer your questions, but if you have any doubts, please come back to me. Also, if you do any of the tests/strategies I mention here, I would like to know the result to keep supporting you in this question.
Many thanks again for reading The Living Tank and your questions! I am here at your disposal.
Best wishes!